Respiratory protective equipment intended for medical use.
A medical face mask with an adequate microbial barrier can also be effective in reducing the emission of infective agents from the nose and mouth of an asymptomatic carrier or a patient with clinical symptoms.
NEN-EN 14683:2019 + C1:2019 SPECIFIES CONSTRUCTION, DESIGN, PERFORMANCE REQUIREMENTS AND TEST METHODS FOR MEDICAL FACE MASKS INTENDED TO LIMIT THE TRANSMISSION OF INFECTIVE AGENTS FROM STAFF TO PATIENTS DURING SURGICAL PROCEDURES AND IN OTHER MEDICAL SETTINGS WITH SIMILAR REQUIREMENTS
This European Standard is not applicable to masks intended exclusively for the personal protection of staff. The standard defines surgical masks as: medical devices, covering the mouth, nose and chin that form a barrier to limit the transition of an infectious agent between the hospital staff and patients.
Regarding performance, the mask is tested as a final product and must comply with specific requirements.
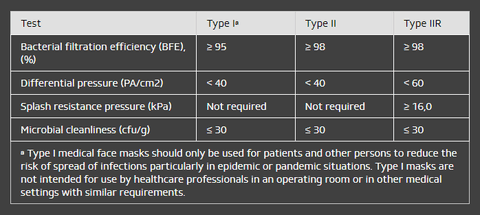
The classification of masks is based on results from the following tests:
- Bacterial filtration efficiency (BFE)
- Breathability (delta P)
- Splash resistance (synthetic blood)
- Microbial cleanliness
- Biocompatibility
The medical face masks described in this European Standard are classified as Type I and Type II depending on their bacterial filtration efficiencies. Type II masks are subdivided into masks that are and are not splash-resistant (“R” signifies splash resistance).
Since surgical masks are considered Type I medical devices, mask manufacturers must perform a
risk analysis and, if needed, additional tests to ensure mask compliance with European Medical
Device Regulation 2017/745.
There are no requirements specifying that masks must be barriers against inert particles.
Summary of performance requirements for medical face masks: